
We partner with leaders across the Research Community, Life Sciences, Healthcare and MedTech Industry to succeed in our vision to advance healthcare with continuous patient monitoring. Our advanced Research Platform speeds up the creation of biomarkers and the development of Medical Digital Therapies.
Research
Accelerate Clinical Trials And Develop Life-Saving Therapies Faster

Corsano Health revolutionizes healthcare with its compact and lightweight medical patient wristband, backed by peer reviewed clinical validation and EU-MDR medical certification (FDA pending). Our AI analytics generate unparalleled insights for researchers and healthcare professionals.
Vision
Advance Healthcare With Continuous Patient Monitoring

Corsano provides continuous Ambulatory Monitoring that reduces healthcare expenses, improves clinical outcomes, and enhances patient experiences. Our CardioWatch System detects early patient deterioration and impending rehospitalization with a predictive accuracy comparable to implanted devices.
Patient Care
Lower Overall Healthcare Costs And Improve Clinical Outcomes
Continuous Patient Monitoring.
Anytime, Anywhere.
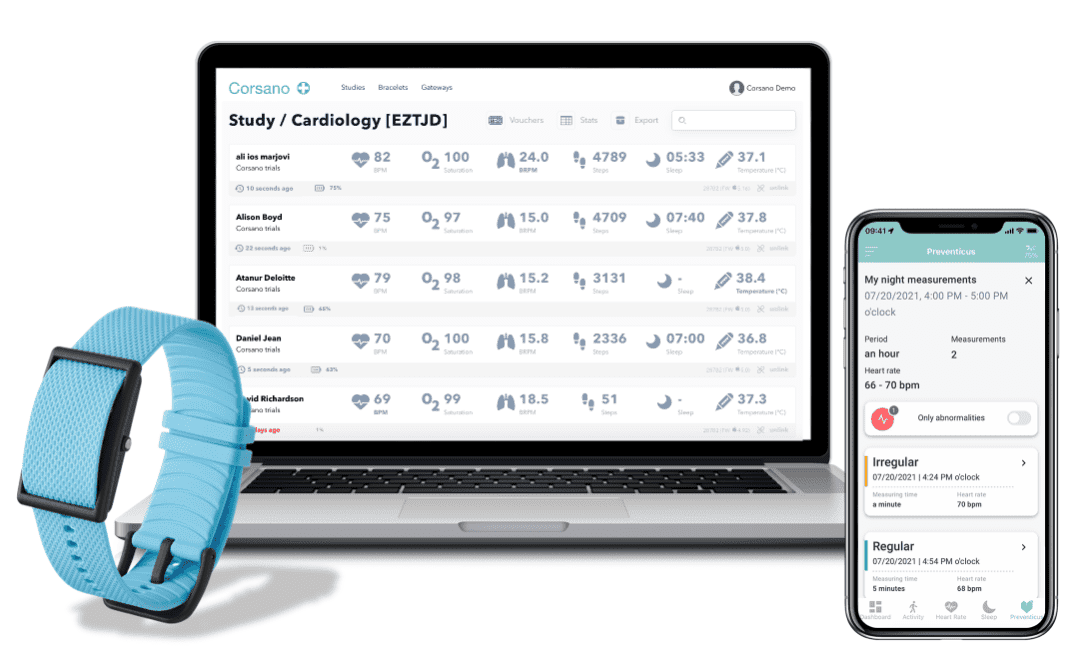
Real time patient monitoring platform, enabled by the world’s most advanced smart bracelet for continuous monitoring of vital signs, with un-restricted access to raw data for AI and ML development. AI analytics that generate unparalleled insights for researchers and healthcare professionals.
Clinical Validation
Validation of a novel cuffless
photoplethysmography-based
wristband for measuring
blood pressure according
to the regulatory standard
Chronic hypertension is a significant risk factor for cardiovascular diseases (CVD) [1]. However, obtaining reliable and reproducible cuff blood pressure (BP) measurements is challenging

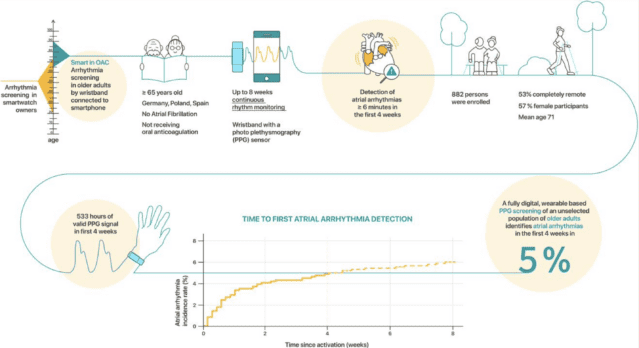
Smartphone and wearable
detected atrial arrhythmias
in Older Adults:
Results of a fully digital
European Case
finding study
The fully remote, investigator-initiated Smartphone and Corsano Health’s CardioWatch 287 wearable detected atrial arrhythmia ...
Clinical Validation
The accuracy of heartbeat
detection using
photoplethysmography
technology in cardiac
patients
This validation study showed that the Corsano 287 CardioWatch/Bracelet with PPG-technology can determine HR and RR-intervals with high accuracy in a cardiovascular patient population...
Clinical Validation

Corsano
est. 2019
Data Points
Devices Produced
Hospitals
Patients
Clinical Trials
Corsano CardioWatch
Cardiowatch is a wireless remote monitoring system intended for continuous collection of physiological data in healthcare and home settings. Cardiowatch is a CE medical device certified under EU-MDR standards and FDA 510(k) Cleared. This includes Pulse Rate, Heart Rate Variability (R-R intervals), ECG, SpO2, Respiration, Blood Pressure, Core Body Temperature, Activity and Sleep. Data is transmitted wirelessly from the bracelet via the application or gateway to a health cloud where it is stored and made available for further analysis.
Corsano Cardiowatch can include the ability to notify healthcare professionals when physiological data fall outside selected parameters.
Medically
CE-MDR Medical Certification
FDA 510(k) Cleared
Validated Vital Sign algorithms
Proven platform in clinical research and patient care
State-of-the-art biomarker development: Hypertension, Cardiac Arrest, Immune Oncology, Frailty, Scepsis
Certified






Patient
Centric
Smallest and lightest patient
bracelet in the world
Long battery life
Single purpose device for long-term patient monitoring
Very easy to use
Proven with 10'000+ patients, incl. senior population and children






Research
Portal
Best practices security,
GDPR and HIPAA
Full flexibility to create studies, set parameters and sampling frequency
Multi parameter analytics
Compliance metrics
Easily export data
Data science support






Raw
Data
Multi sensor:
ACC
PPG G/R/iR
ECG
Temperature
GSR
Granular high resolution Vital Signs and raw data available to researchers
Develop new algorithm with IP free data




Integration with any platform via APIs
Implementation research pipeline with automatic sync
Third party apps may pair and sync directly CardioWatch Bracelets via SDKs
Communicate directly with EPDs

Integration




Clinical Trials
Corsano is enabling clinical trials, in-hospital and remote patient care across the entire care continuum with our clinically validated and EU-MDR and FDA certified CardioWatch 287 System.
Corsano is currently involved in over 100 clinical trials – Cardiovascular, Oncology and Stress. Corsano’s medically certified wearables increase credibility of studies and prepare for medical digital therapies. Corsano’s involvement in important clinical trial research consortia accelerates the pace of medical research, enabling the discovery of new biomarkers and their clinical validation.
Real-time Remote Patient Monitoring
Corsano CardioWatch 287 can monitor up to 19 Vital Parameters in real-time while post-discharge programs often rely solely on episodic vitals (spot measurements). Healthcare providers can develop personalized treatment plans that address the unique needs of each patient. Corsano can provide patients with real-time feedback on their progress, which can help motivate them to adhere to their treatment plan. Remote care-at-home programs deliver improved clinical outcomes, lower costs and better patient experience: 38% reduction in cost of care; 70% reduction in readmissions; 50% improvement in patient mobility
In-Hospital Monitoring
This is the solution where data from CardioWatch is directly streamed to the gateway (no need for phone app) and transferred to the hospital IT system, enabling central monitoring of patients and improved quality of work for nurses. By providing nurses with real-time information about their patients’ conditions, central monitoring can help ensure that patients receive appropriate care, treatment, and medication at the right time, leading to better overall outcomes.
Corsano offers an API (Application Programming Interface) and SDK (Software Development Kit) to providers of remote patient monitoring platforms and researchers, which allows them to connect Corsano CardioWatch to their platform. The API and SDK enable to access the data collected by Corsano CardioWatch, including synching of raw PPG, ECG, ACC and BIOZ data.

Uninterrupted Ambulatory Monitoring
Remote Patient Monitoring
CardioWatch is the smallest multi sensor medical bracelet to provide care in a patient’s home though continuous, remote monitoring
Detect impending rehospitalization with a predictive accuracy comparable to implanted devices
Studies indicate that patients experience improved outcomes with hospital-at-home services compared to conventional hospital stays


Early detection of patient
deterioration
In-Hospital Monitoring
Patient deterioration between routine nurse observation rounds can go undetected for hours, resulting in severe and expensive outcomes.
Corsano’s monitoring and smart algorithms provide reliable data to support clinical decisions and detect the earliest signs of patient deterioration.

Testimonials
First, I wanted to let you and the Corsano team know how very impressed I am with your device. I’ve tried out more wearable sensors than most people, and the CW287-2B is, by far, my favorite and most valuable.
Congratulations to your entire team for your vision and for delivering on that. I believe your technology will have a significant impact on improving health across the globe.
Prof. Steven Steinhubl
MD Purdue University
As a part of the DETECT consortium, our collaboration with Corsano has been instrumental in advancing technological solutions aimed at the early detection of cardiac arrest. This partnership is paving the way for significant breakthroughs that we believe will greatly enhance patient care and outcomes.
Niels van Royen, MD, PhD
Professor of Cardiology, Head of Department, Radboud University Medical Center
Corsano understands the challenges we face in care, related to care burden and costs. CardioWatch offers automated vital signs monitoring and integrates data into health records, easing our nursing staff's workload. Our hospital effectively addressed implementation areas like data security and legalities. We're very pleased with our Corsano partnership.
Dr. Eelko Ronner, MD, PhD
Cardiologist, Delft, Netherlands
Our Partners
Corsano has made “Cooperation” one of the core values upon which the company commits to base all its relationships. To be successful in business and life, a relationship has to be a two way street with give and take. At Corsano, we believe in cultivating good working relationships with our partners. We don’t limit ourselves to a select few, but always attempt to find the best possible partners.
Corsano develops and manufactures medical wearables for research and patient care, that is our core business. Our open strategy is to connect CardioWatch to partner platforms and systems via APIs and SDKs. We provide access to raw and processed data to partners enabling them to offer a variety of medical research and patient care services. As such, we deal with hospitals, CROs, associations and companies worldwide.
Large Research Projects
Corsano participates in Europe’s research and innovation challenges where we collaborate with researchers in consortia with partners.
Corsano Health’s CardioWatch is the preferred choice to measure vital parameters, both for the acquisition of raw data up to 128Hz, as well as validation clinical trials.
We offer co-development of new features and algorithms to increase success of consortia applications. Example of awarded projects:

LifeTime
Project for a validated, energetically autonomous module that harvests from any source of light through a novel microchip, the PMIC developed by Nowi. Narrow-Band IoT chip (NB-IoT) will be integrated into the module for ubiquitous connectivity.

Watch-It
Clinical validation of a comprehensive care solution (‘WATCH-IT’) enabling 24/7 monitoring of cardio-respiratory function in patients at risk of cardiovascular (CV) events. Collaboration with Preventicus, Oslo University Hospital (OUH) and University of Gothenburg (GU).

Detect
Innovative solution to improve recognition and implementation of resuscitation assistance for patients with Cardiac Arrest. Development project in colaboration with RadboudMC, ErasmusMC and UMC to recognize Cardiac Arrest with Cardiowatch 287 Bracelet and alert emergency services.
































